DM² 1ˢᵗ-Gen Demonstrators
The Made Smarter Innovation - Digital Medicines Manufacturing Research Centre (DM²), led by CMAC, is reshaping medicine manufacturing by addressing traditional inefficiencies.
Co-funded by the Made Smarter Innovation Challenge at UK Research and Innovation and industry partners, DM² recognises that data and industrial digital technologies (IDTs) are essential for the development of integrated digital solutions.
DM² strives to decrease waste, boost efficiency, foster a future-ready workforce, and enhance the responsiveness of the medicines manufacturing industry for faster, sustainable delivery of medicines to patients. Through collaboration with industry and academic partners, DM² co-creates and co-delivers innovations aimed at improving patient outcomes and ensuring the industry's readiness for the future.
The DM² team is working over several integrated delivery Platforms to achieve their goals. To mark the project’s mid-point, the team recently shared their progress at a showcase during CMAC Open Days 2023:
13 Demonstrators
Across Data, Autonomous Manufacturing, Quality Control Tools, Supply Chain, Engagement and Skills
7 Integrated IDTs
Industrial digital technology developments covering data collation & transformation, machine learning/AI, computer modelling, robotics, digital design and immersive tech
300* Attendees
Across 3 showcase sessions
* approx. 300 attendees present across 3 days of CMAC Open Days
Platform 1:
The team’s research focuses on data organisation, aiming for data to be Findable, Accessible, Interoperable, and Reusable (FAIR) to enhance its usability in manufacturing. In the first half of the project, the team have developed onotolgy mechanisms to extract biomedical relations from text and Extract, Transform and Load (ETL) tools to simplify data acquisition, databasing and visualisation. Platform 1 has also been involved in the creaion of an automated sessile drop experiment for the real-time testing of tablet disintegration properties. This is an integral part of the testing mechanisms being integrated into the Platform 2 automated micrcoscale tabletting and testing workflow.
Watch the video or click on the posters to learn more about Platform 1’s research.
Platform 2:
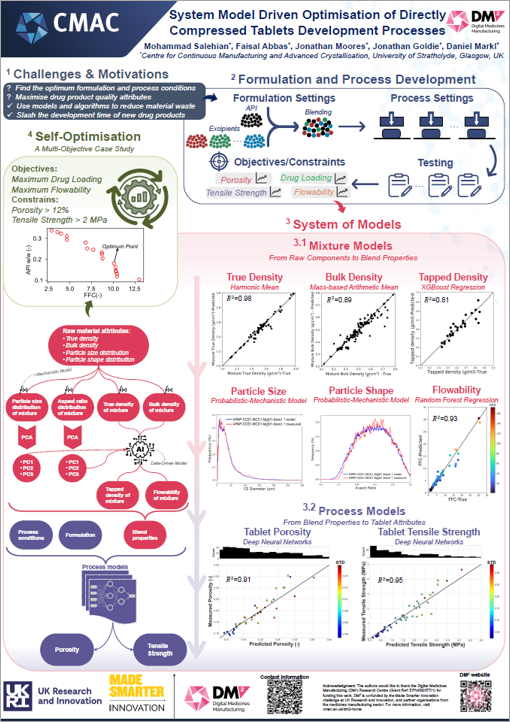
The team has been working on an autonomous microscale production facility, utilising AI, robotics and computational modelling. The team have created a small-scale manufacturing set-up integrating several technologies and instruments to make and test individual medicine tablets. This has the capacity to run independently on user-defined and/or self-driven experiments. Taking an integrated approach, driven and optimised by computational models and machine learning, helps identify the best formulation and process conditions to maxisimise drug product quality attributes, reduce waste and cut development time.
Watch the video or click on the posters to learn more about Platform 2’s research.
Platform 3:
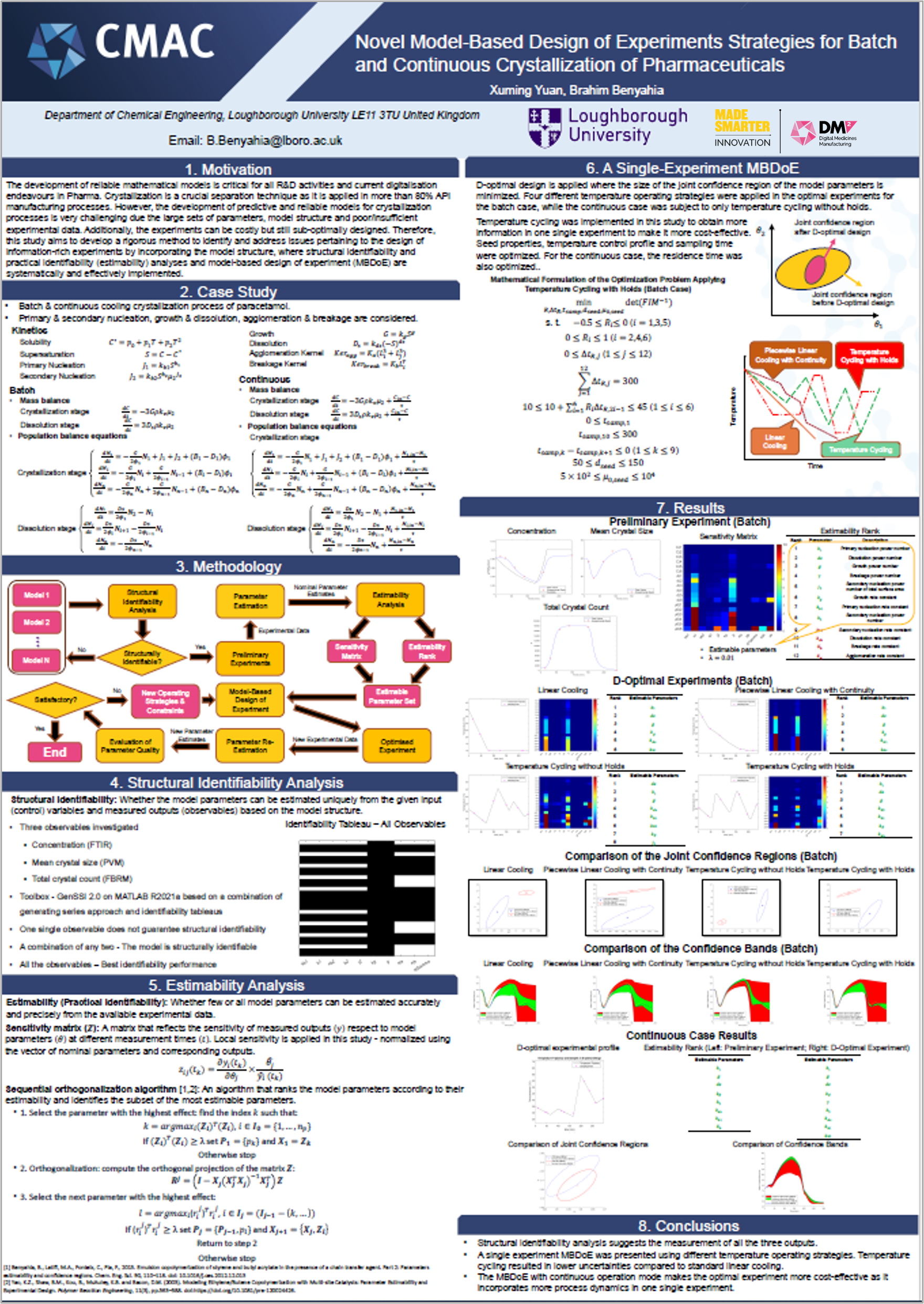
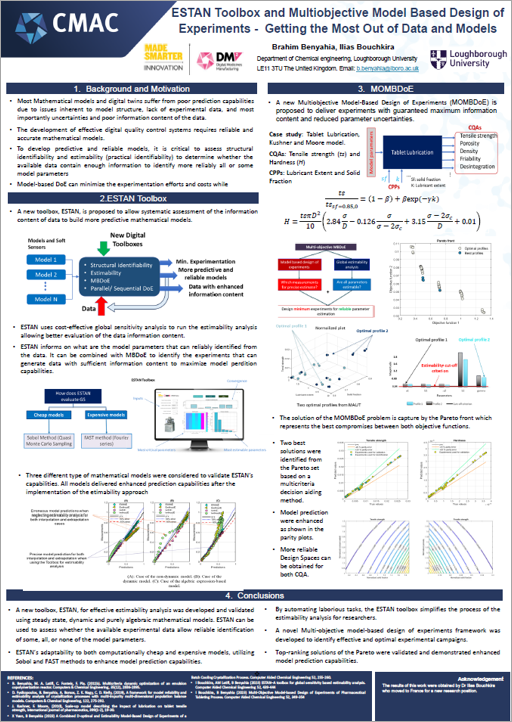
The team’s research is driving digitalisation of quality control processes by developing digital design tools that streamline medicines manufacturing. These are focused on ensuring safe medicine release by creating data-driven, integrated safety and compliance checks in real-time. To achieve this, the team has created a toolbox named ESTAN to get the most out of data and models, increase the reliability of model-based design of experiments and realise cost and time saving benefits. For similar reasons, the team is also running an investigation into quality control mechanisms for a low-dose capsule filling process, using Discrete Element Methods (DEM). Exploring how planning and scheduling of clinical trials can be informed by data generated through this digital quality control research has also been initiated by the team.
Watch the video or click on the posters to learn more about Platform 3’s research.
Platform 4:
The team is investigating the impact of digital interventions on creating an adaptive supply network that responds to changing materials supply and healthcare provider needs. A number of use cases have been initiated with project partners in the healthcare sector. The first of these cases is centred on digital platform developments, connecting pharmaceutical and medical device manufacturing operations to downstream healthcare product procurement systems to enhance availability. Another case seeks to make clinical trials more resiliant and effective by leveraging extensive analysis of clinical trial supply chain data, and investigating digital interventions to prevent stock-out events. Thirdly, the team is researching production and logistics synchronisation for smart pharmaceutical manufacturing to streamline processes, enhance efficiency, improve stocks and reduce waiting times and operational costs.
Watch the video or click on the posters to learn more about Platform 4’s research.
Platform 5:
The team leads stakeholder engagement and collaboration, and has been running a schedule of activities such as webinars, workshops and conference events to maintain and grow an informed, diverse network of medicines manufatcturing stakeholders. Engagement is critical to building trust in new Industrial Digital Technologies (IDTs) and nurturing an appetite for their implementation and the beneficial changes they will bring. Critical to this is a confident and sufficiently skilled workforce, ready to implement and work alongside new IDTs. To facilitate this, the team has established a SkillsFactory, integrating immersive tech like Augmented Reality, to prepare the workforce for the digital era.
Watch the video or click on the posters to learn more about Platform 5’s research.